Companion diagnostics
Companion diagnostics is a clinical test co-developed with a therapeutic drug to screen responder to particular medications. It is done to identify a group of patients who may or cannot be treated with the drug. Companion diagnostics ensures patient safety by helping doctors determine the benefits, side effects, and risks associated with consuming a therapeutic drug.
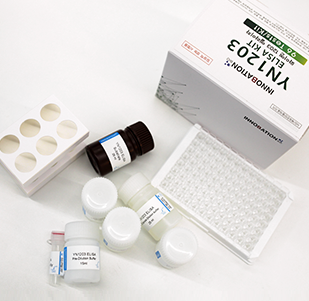
YN1203, InnoBation Bio’s companion biomarker applicable on prediction of response of immune checkpoint inhibitors, shows its excellence in its non-invasivity, accuracy, real time assay, versatility, and common operation.
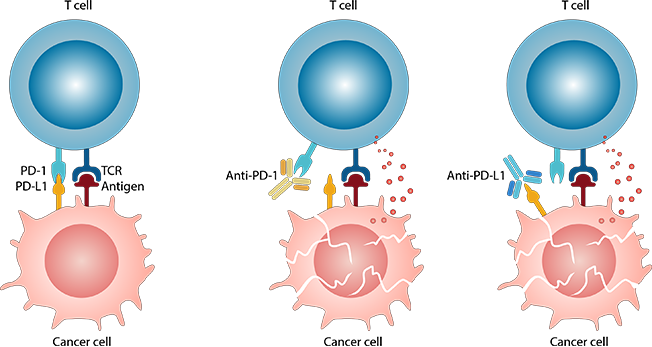
To avoid autoimmune disorders, our immune system has immune checkpoint molecules, for example, PD-1/PD-L1. The bad news is that tumor cells also use these checkpoint molecules to evade T cell-mediated immune surveillance, which is one of the important immune cells that play a key role in preventing cancer development and progression. Inhibiting these immune checkpoint molecules to help T cells recognize tumor cells is the major type of cancer immunotherapy.
Companion diagnostics are essentially required before these immune checkpoint inhibitors are used for cancer patients to pre-check their efficacy and safety.
There are many FDA-approved immune checkpoint inhibitors in the market, mainly targeting PD-1/ PD-L1. PD-L1 expression on tumor cells has been thus far widely used as a predictive biomarker for PD-1/PD-L1 blockade. However, they often show low efficacy due to differential expressions among cancer types.
In 2019, FDA claimed that the use of PD-L1 expression as a predictive biomarker has limitations and that the decision to pursue testing must be carefully implemented for clinical decision making.
Companion diagnostics biomarker
Our team found that YN1203 can be used as marvelous companion biomarker for predicting the response of immune checkpoint inhibitors. The trials were performed on non-small cell lung cancer (NSCLC) patients treated with 3 different types of immune checkpoint inhibitors. Plasma samples were collected from a total of 169 NSCLC patents before the treatment of immune checkpoint inhibitors.
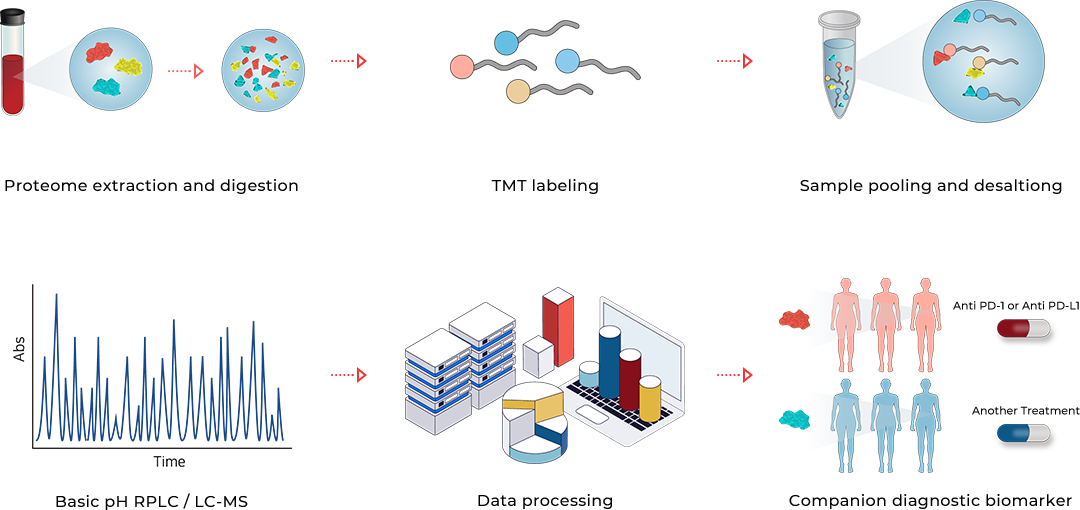
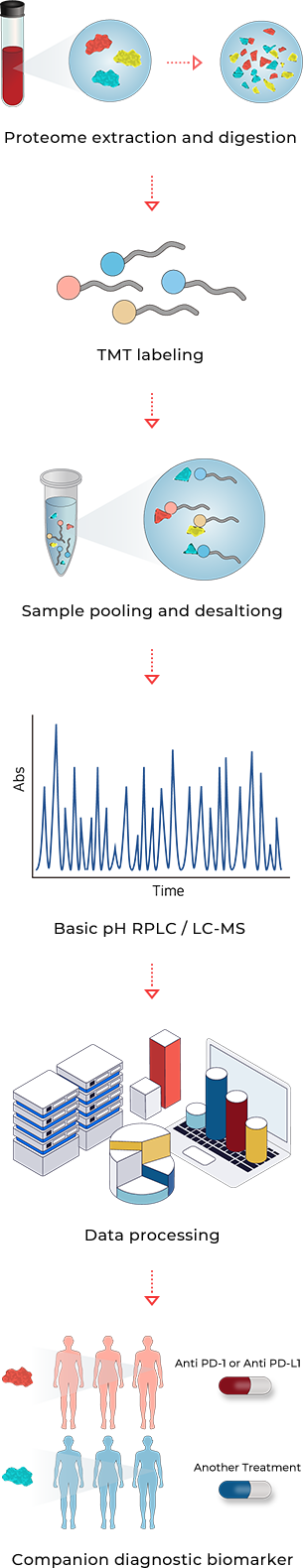
YN1203
YN1203 has been quantified in 169 plasma samples and determined as the optimal companion diagnostic biomarker for separating responder and non-responder group through diverse statistical analyzes to validate analytical performance.
YN1203’s outstanding predictive power has been confirmed by comparison with other products.
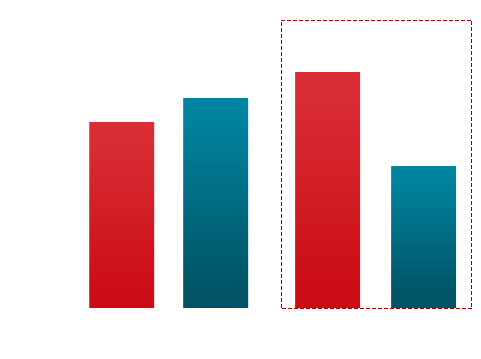
In its comparative experiment with currently wide used companion diagnostics tests (PD-L1 IHC CDx A & B) YN1203 show 21% higher accuracy than PD-L1 IHC CDx A and 19% higher than PD-L1 IHC CDx8 under PD-L1 TPS <50 % NSCLC condition. Further ROC (Receiver Operatoing Characteristics Curve) analysis in same condition shows AUC (Area Under the ROC Curve) value of YN1203 is 28% higher than PD-L! IHC CDx A and 17% higher than PD-L1 IHC CDx B.
Quantification of YN1203 has been performed twice and mean amount value of YN1203 was used for calculating each predictive powers. Results obtained from the multicenter retrospective study are summarized in the figures.
YN1203 vs PD-L1 IHC CDxA
 YN1203
YN1203
 PD-L1 IHC CDxA
PD-L1 IHC CDxA
PD-L1 TPS (%) < 50%
 YN1203 (AUC = 79.5%)
YN1203 (AUC = 79.5%)
 PD-L1 IHC CDxA (AUC = 51.7%)
PD-L1 IHC CDxA (AUC = 51.7%)
 Ref. line
Ref. line
YN1203 vs PD-L1 IHC CDxB
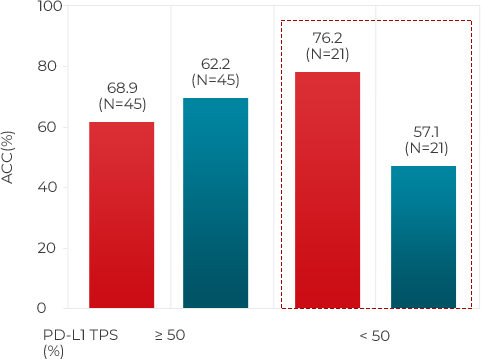
 YN1203
YN1203
 PD-L1 IHC CDxB
PD-L1 IHC CDxB
PD-L1 TPS (%) < 50%
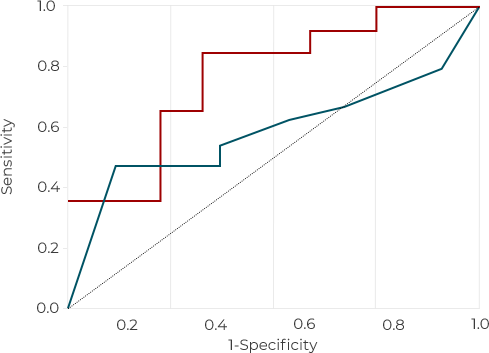
 YN1203 (AUC = 75.9%)
YN1203 (AUC = 75.9%)
 PD-L1 IHC CDxB (AUC = 58.3%)
PD-L1 IHC CDxB (AUC = 58.3%)
 Ref. line
Ref. line
InnoBation Bio’s YN1203 ELISA kit provides accurate predictions of immune checkpoint inhibitors response on patients using YN1203 as biomarker.

Through the multicenter validation and exploratory clinical trial, YN1203 has been proven as a powerful biomarker to predict the response of immune checkpoint inhibitors.