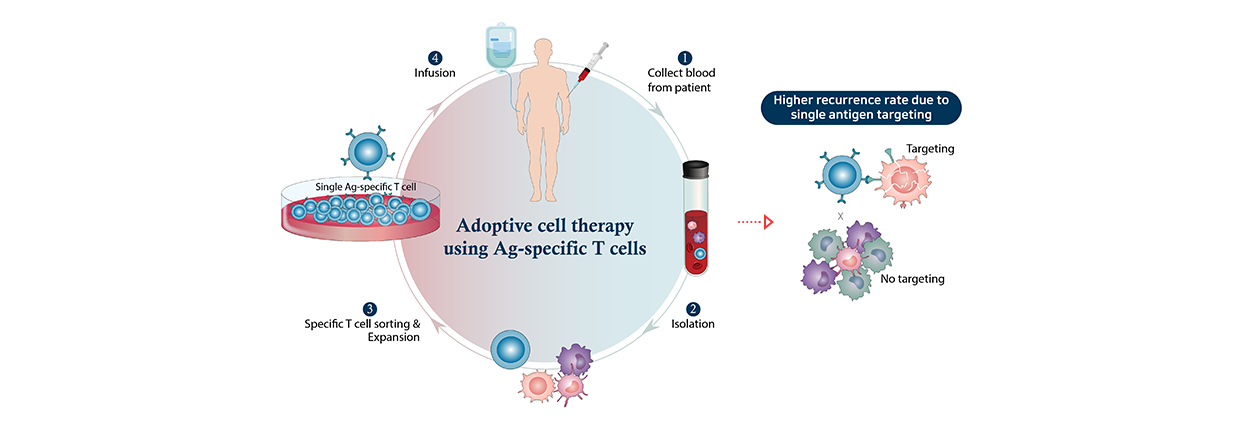
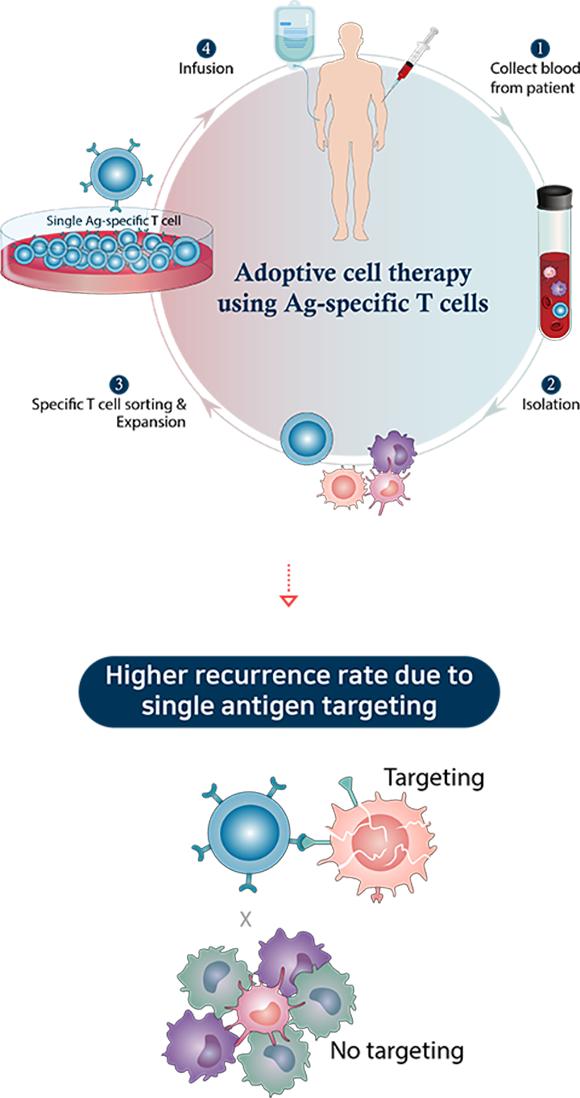
Adoptive immune cell therapy using T cells
Adoptive immune cell therapy using T cells is a treatment that induces the therapeutic effect by re-administering a cancer patient's immune cells after expanding them ex vivo. Among various immune cells, anti-cancer immunotherapy using T cells is considered a safe and high potential next-generation cancer therapy. But this type of cell therapy shows limited therapeutic effect in solid cancers.
Existing anti-cancer T-cell therapy products target a single cancer antigen for product standardization which increases the likelihood of recurrence. Additionally, the production process requires the isolation of T cells from patients' blood in which they are present in extremely low proportion. This complicates the manufacturing process. The recently developed MapT (mass production of T cells targeting multiple tumor antigens) therapy is a technology for culturing and expanding T cells targeting multiple cancer antigens in large quantities and is expected to increase anti-cancer effects and lower the recurrence rate.
MapT
Therapy
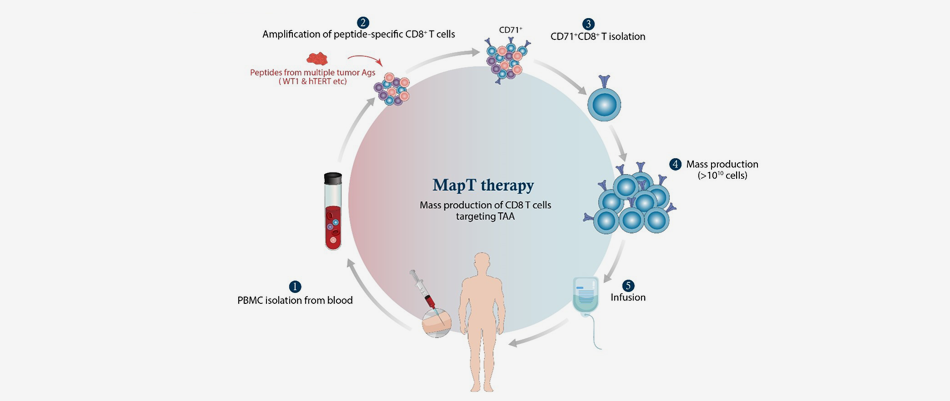
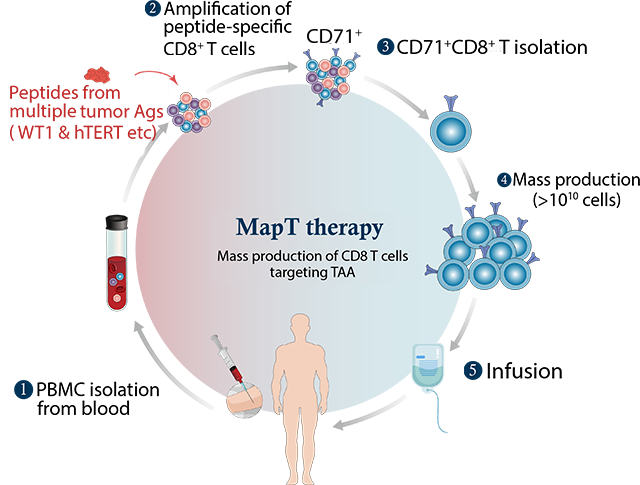
MapT therapy is a platform technology that enables the development of product groups targeting various cancer antigens. MapT cells are produced by isolating and expanding CD71-expressing CD8+ T cells following stimulation of T cells with peptides originating from tumor-associated antigens like Wilms’ Tumor 1 (WT1) and human telomerase reverse transcriptase (hTERT), which includes peptide-specific CD8+ T cells and some of spontaneously expanded CD8+ T cells in response to tumor growth. MapT cells are designed to target multiple tumor antigens and thus expected to reduce the recurrence rate after the treatment.
The pilot production and stability test of MapT-WT cells, the first product of MapT therapy that mainly targets WT-1, which is a suitable cancer antigen for the treatment of several cancer types including glioblastoma and ovarian cancer, has recently been completed and preparing to apply for Phase I clinical trial approval.
A phase I clinical trial to evaluate the safety of MapT-WT cells in single-center, open-label, single-group, and recurrent advanced solid cancer patients is scheduled next year after the Ministry of Food and Drug Safety grants the clinical trial approval.